Services
DiGA (Digital Health Applications)
The focus is on the goal of listing your DiGA (digital health application) and the resulting reimbursement eligibility.
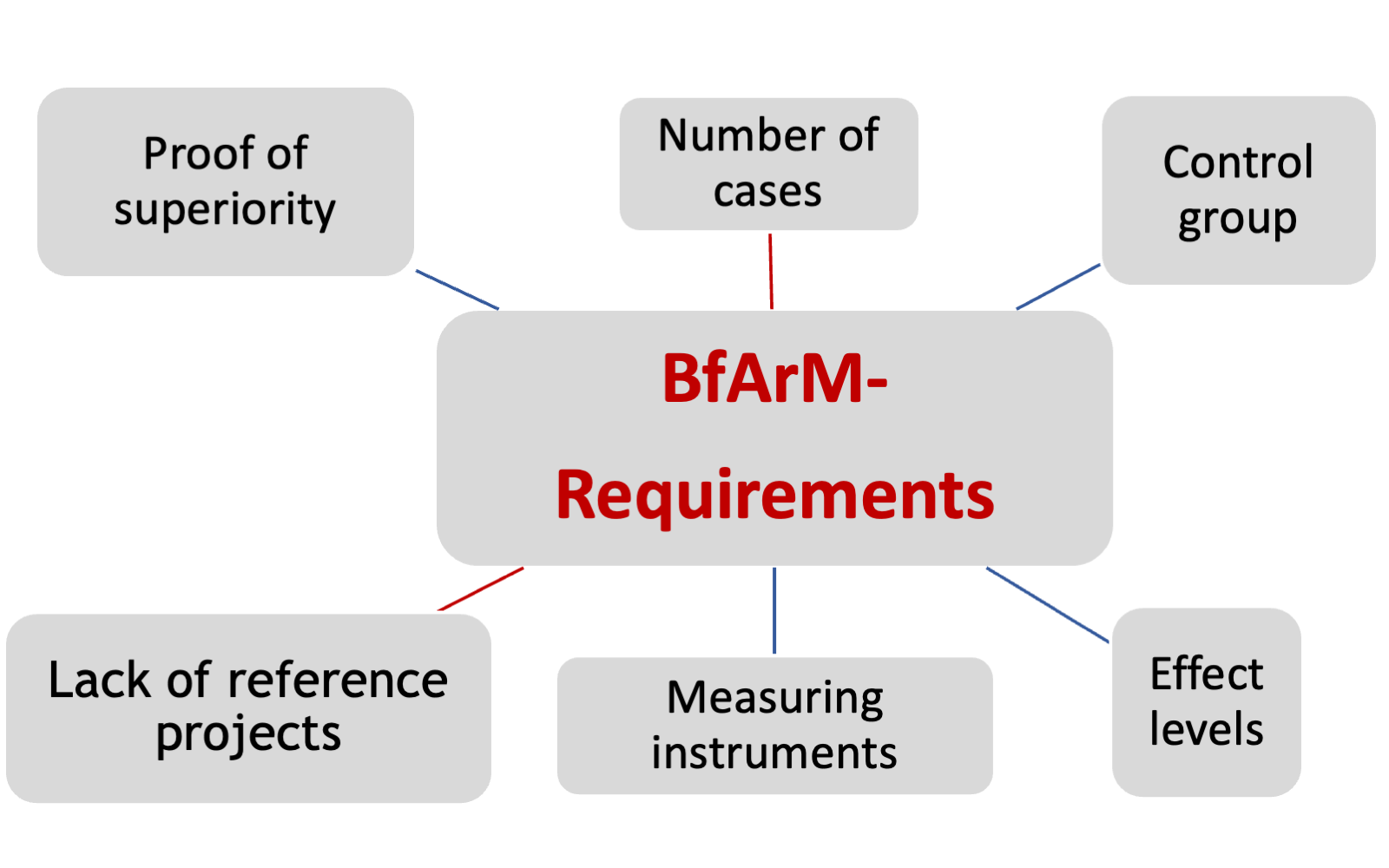
The DiGA process begins in the post-market phase of your CE-marked medical device. We will also be happy to support and advise you on the way to CE marking! (see Regulatory Affairs & QM) You must provide proof of positive care effects (pVE) for your product: “DiGA manufacturers who submit an application for testing must plausibly demonstrate that the DiGA can achieve one or more pVE for a specific patient group .
This requires the submission of a systematic evaluation of data for the use of the DiGA.” (Source: current BfArM guidelines) You can generate the data for the proof in various ways; we will support you in choosing the optimal design. We design the DiGA studies in such a way that the requirements of DGV, DiGAV and guidelines are met, but the clinical data can also be used for clinical evaluation in accordance with the MDR requirements.
The provisional listing
- Supporting the concept for systematic data collection as input for the evaluation concept
- Advice and support regarding possible BfArM discussions
- Systematic data evaluation
- Creation of the evaluation concept (including targeted literature research and evaluation in accordance with the BfArM guidelines)
The final listing
- Planning the DiGA study
- Preparation of all required documents in accordance with ISO 14155
- Implementation of the DiGA study including data management and monitoring
- (statistical) evaluation of the study data and creation of the clinical investigation report
-> You can find our offer in the context of clinical investigations here
