Literature search for medical devices
At medXteam, the focus is on clinical data. In this context, as CRO we not only carry out clinical trials with medical devices in accordance with MDR and ISO 14155, but also offer all other options and forms of data collection. The literature search plays an important role in this context. Because it is not only essential in connection with the clinical evaluation of medical devices. This blog post shows when, where and why you need a literature search in other situations.
Abbreviations
MDR Medical Device Regulation; EU Regulation 2017/745
DiGA Digital Health Application
PMCF Post-Market Clinical Follow-up, clinical follow-up
QMS quality management system
Underlying regulations
EU Regulation 2017/745 (MDR)
Medical Device Implementation Act (MPDG)
ISO 14155
1 Introduction
In the regulatory environment of medical devices, the literature search is an elementary process that takes place in different phases of the life cycle. It is relevant not only in clinical assessment, but also in context
- the clinical strategy,
- the development strategy,
- of risk management
- in connection with clinical trials, PMCF studies
- at DiGAs with regard to systematic data collection
This article therefore first answers the question: When do I carry out a literature search for medical devices? The different contextual situations and the design of the search in the respective situation are discussed.
In addition, we also show how to conduct an effective literature search and offer practical examples for designing the search strategy in different situations.
2. Literature search and clinical data
When it comes to the topic of “literature search”, clinical data obviously plays an important role. Clinical data is the heart of medical research and refers to the collection of information about the product when used on humans. They include a wide range of information obtained from clinical studies, patient observations, research results and other medical sources. This data is essential for assessing the effectiveness, safety and quality of medical devices. They form the basis for regulatory decisions and make a significant contribution to the development of innovative medical solutions.
Clinical data is defined in connection with medical devices in the MDR in Art. 2 as follows:
“Clinical data” means safety or performance information obtained through the use of a product from the following sources:
- clinical trial(s) of the product in question,
- clinical trial(s) or other studies reported in the scientific literature on a product whose similarity to the product in question can be demonstrated,
- in peer-reviewed scientific literature published reports of other clinical experience either with the device in question or with a device whose similarity to the device in question can be demonstrated,
- clinically relevant information from post-marketing surveillance, in particular from post-marketing clinical follow-up."
The literature search is therefore the process of finding clinical data. This leads us into the world of medical databases. The most important sources include PubMed, the Cochrane Library and EMBASE. These databases provide access to a variety of publications, journals, conference proceedings and systematic reviews, meta-analyses, guidelines and much more.
The literature search process takes place in several steps and is the same in every regulatory situation:
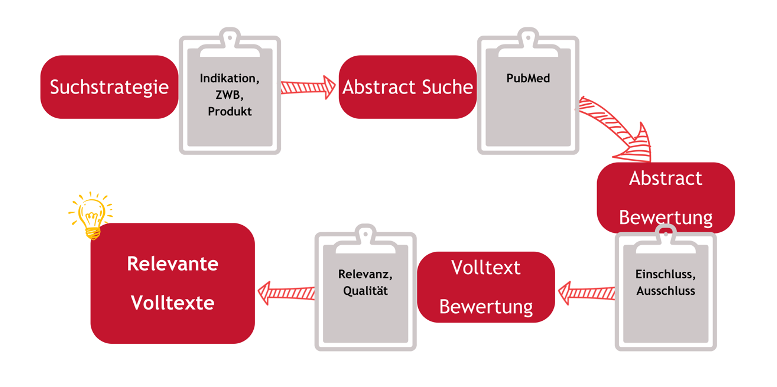
Fig. 1: Literature search process
Defining the search strategy : The first step is to carefully plan the search strategy. Relevant keywords and search terms are defined that form the framework of the research.
Choosing the right databases : Because of the wealth of information, choosing the right databases is crucial. Each database has its own strengths and specializations that need to be taken into account.
Carrying out the search : The databases are systematically searched using the defined keywords. This phase requires patience and care to ensure that no relevant information is missed.
Analysis and selection of data : After collecting the information, the results are critically evaluated. The most relevant and well-founded studies and reports are selected that help answer the question.
A possible technique that can be used in this process is e.g. B. the PICO technique: It helps to make search queries more precise and more effective. PICO stands for Population, Intervention, Comparison and Outcome. This method makes it possible to focus the research on the most important aspects, thereby providing more precise and relevant results.
This technology is used in particular in the context of
- Patient care
- Treatment
and for determination
- the accuracy of diagnostic tests
- prognostic factors
used.
3. Literature search in practice
The literature search enables well-founded decisions to be made, in this context we also speak of “evidence-based” decisions. It is therefore an indispensable part of the development and evaluation of medical devices because it
- provides a structured basis for decision-making and
- which ensures the quality, clinical performance and safety of medical devices.
There are various situations in the product life cycle of a medical device in which a literature search is necessary. Each has specific goals and foci:
- Context Clinical Assessment: Plan, Report
- Context Clinical strategy, development strategy, risk management
- Context Clinical trial, PMCF study, systematic data collection
These are discussed in detail below.
3.1 The literature search in the context of clinical evaluation
The clinical evaluation of medical devices (Article 61 of EU Regulation 2017/745 (MDR)) is a core element of technical documentation and, as part of the validation of clinical data, confirms the safety of the medical device, its clinical performance and its benefit-risk ratio. The literature search is an integral part of providing this information. The process of "assessing" clinical data is a defined sequence of actions to analyze the various sources, including clinical trials, not only in terms of content but also methodologically. Evaluation criteria include the relevance of the publication, the quality and scientific validity as well as the weighting of the data with regard to the clinical evaluation.
The analysis of “state of the art” data captures the current state of the art. In contrast, the data of the product being evaluated is provided to substantiate the claimed clinical performance and safety of the product.
The literature search includes four steps:
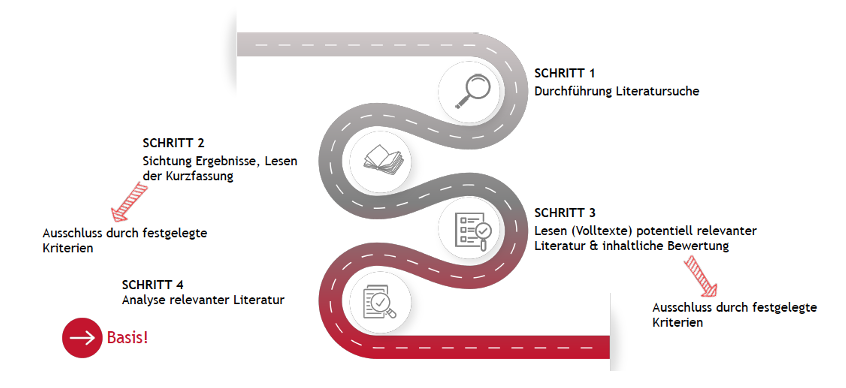
Fig. 2: Literature search step by step
The aim of the clinical evaluation is to create a sound basis for market approval and to ensure the quality, safety and effectiveness of the medical device. This requires careful documentation of the entire process, including literature search plan, protocol and report, to ensure transparency and traceability for audits and regulatory reviews.
3.2 Context clinical strategy, development strategy, risk management
Clinical strategy:
In these areas, literature searches facilitate the identification of potential risks and the development of risk mitigation strategies. It also supports the formulation of a long-term clinical and development strategy based on current research and existing data. It thus lays the foundation for the clinical evaluation route and sets the course for the entire planning of the development process in terms of costs and time.
Risk management:
Risk management is the systematic application of management strategies to identify and control product risks. There is a close interface between risk management and clinical assessment, especially when incorporating the current medical and technological state of the art.
The literature search in the context of the clinical strategy is also carried out in four steps (see Figure 2).
A focus is on searching for similar products to assess equivalence, identify side effects and incorporate market data. The product's intended use is also examined, including the prevalence and incidence of relevant conditions or diseases, alternative forms of use, and current medical guidelines.
3.3 Context clinical trial, PMCF study, systematic data collection
Clinical test:
The clinical trial is designed in the project planning phase and carried out with the final product as part of product validation. The collected data flows into the Clinical Evaluation Report (CER) and is crucial for the product's market entry. It is carried out in accordance with legal requirements such as MDR and ISO 14155.
The literature search in the context of clinical testing is again carried out in four steps (see Figure 2). The focus here is on identifying relevant endpoints on the basis of which the research question should be answered. Furthermore, ideas for a potential study design should be collected.
DiGA:
For digital health applications (DiGAs), a literature search for the "minimally important difference/change" (MCID) is crucial for the systematic data collection and evaluation of the data collected on the product in order to assess the clinical significance of the data and classify it accordingly. But the DiGA guidelines require a systematic literature search, particularly for the evaluation concept: it should provide evidence of the positive care effect.
4. Digital literature search
We have seen how important and central the literature search is in connection with medical devices, across the entire product life cycle.
medXteam specializes in the collection and evaluation of clinical data: our focus is on literature searches. The execution of objective research in Pubmed and Pubmed Central can be partially automated with digital software solutions to ensure comprehensible and reproducible research documentation and to reduce the effort required for documenting the research results. The solution used (Polarion with avaPubmed extension) offers a direct, validated interface to Pubmed and Pubmed Central.
4.1 Digitized literature search via Polarion
Literature search is the core process of clinical evaluation.
When searching for literature via Polarion, a direct connection is established to the database sources (e.g. directly to PubMed).
The literature search is carried out and documented in the form of the following documents:
- Literature Search and Review Plan
The literature search and review plan describes the objective search and describes the identification of publications. It includes:
- Sources of publications
- Search terms
- defined filters
- Assessment criteria and process for identified publications
- Process for analyzing the relevant publications
- Literature Search Execution Protocol
The implementation protocol provides details of the research carried out and an overview of the history of the research. It includes:
- Search queries and results used
- Deviations from the literature search and review plan
- Overview of searches carried out and search results
- Literature Review Report
The report contains a summary of the search carried out, as well as the evaluation and analysis. It includes:
- Summary of objective search execution and results
- conducted search and selection procedures for identification by other means
- Evaluation of the identified publications
- Analysis of the relevant publications (see following section)
4.2 Documentation of the analysis
The full text of each potentially relevant publication is read and analyzed with regard to the scope of the literature search and the relevant clinical assessment topics in the respective clinical assessment plan. The extracted statements about safety, performance, benefits, demands or state of the art are documented.
The analysis of a single "publication" is documented in the form of a single "publication evaluation" (see diagram below): The "publication" is linked to the "publication evaluation" and the evaluation is linked to the respective "clinical evaluation object" linked in the clinical evaluation plan. The following graphic explains the connection between the individual work item types:
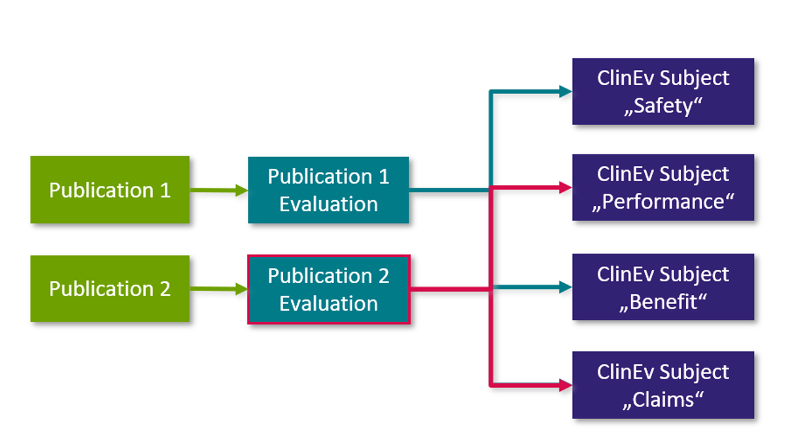
Fig. 3: Analysis
4.3 Report on the literature search
The literature search report provides an overview and summary of the analysis:
For each clinical assessment topic, it is listed which publication was identified as being relevant to this topic and which specific statements were extracted in the publication assessment.
Based on these results, it is analyzed whether the relevant data sets as a whole show evidence for the respective clinical evaluation subject (the respective claim, see figure above). The aim is to look for consistency of results across specific clinical assessment topics. If different results are observed across datasets, it is helpful to determine the reason for these differences.
The following graphic visualizes the connection between the documents and the digital content they contain in the form of work items:
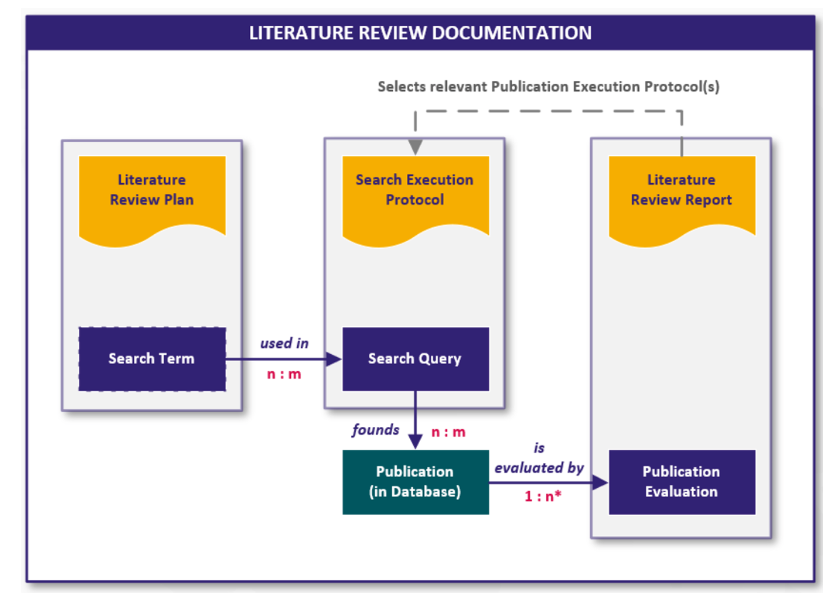
Fig. 4 Interfaces and work items
4.4 Digitalized clinical assessment
Digitalization is of course particularly effective here:
The core of the clinical assessment is the literature search, which can be carried out digitally (see above). Embedded in Polarion as a subsystem, it can also be digitized itself. The following figure provides an overview of the content of the clinical assessment documents
- CEP,
- CERIUM,
- Literature search documents – plan, minutes, report
embedded as a subsystem in the overall technical documentation system:
4.5 Advantages of digitalization
The digitalization of technical documentation for medical devices and thus clinical evaluation and literature search is the future!
The advantages of digitalization are obvious:
- more efficient work
- Target-oriented use of capacities
- Elimination of inefficiencies in the creation, maintenance and modification of technical documentation content, clinical evaluation and literature searches
- long-term reduction in care costs
Via Polarion, interfaces such as purpose, risk management, usability, clinical evaluation, clinical trial can be assigned to projects and reused if necessary. The creation and maintenance of documents is thus significantly simplified and accelerated. In addition, redundancies and inconsistencies are avoided.
5. Conclusion
According to MDR Art. 2, clinical data includes information on safety and performance derived from clinical trials, specialist literature, clinical experience reports and post-market surveillance. These data sources are crucial for an effective literature search. Searching the literature where we find clinical data and the importance of this data to various aspects of medical device development illustrates how fundamental literature searching is to the entire development process.
The literature search for medical devices is more than just a step in the development process; it is a continuous process that has a decisive influence on the quality and safety of medical devices. It enables manufacturers, researchers and clinical experts to make informed decisions based on the latest scientific evidence. In an ever-evolving industry, literature searches remain an essential part of ensuring innovative and safe medical devices.
A literature search is essential for all medical devices over the entire product life cycle, namely: To obtain clinical data!
6. How we can help you
Due to high demand, we have produced a special online training:
This training is designed to provide medical device professionals with comprehensive guidance on effective literature searches in various settings with a focus on clinical evaluation. The training is divided into four lessons, which include both theoretical basics and practical application examples.
Lesson 1: Literature search and clinical data
Lesson 2: Literature search in practice
Lesson 3: Getting started in practice: First practical example
Lesson 4: More practical examples
Clinical trials:
At medXteam we clarify whether and if so which clinical trial needs to be carried out under what conditions and according to what requirements during the pre-study phase: In 3 steps we determine the correct and cost-effective strategy in relation to the clinical trial required in your case Data collection.
If a clinical trial is to be carried out, basic safety and performance requirements must first be met. The data from the clinical trial then feed into the clinical evaluation, which in turn forms the basis for post-market clinical follow-up (PMCF) activities (including a PMCF study).
In addition, all medical device manufacturers require a quality management system (QMS), including when developing Class I products.
We support you throughout your entire project with your medical device, starting with a free initial consultation, help with the introduction of a QM system, study planning and implementation through to technical documentation - always with primary reference to the clinical data on the product: from the beginning to the end End.
Do you already have some initial questions?
You can get a free initial consultation here: free initial consultation
